Partner with Tekton Research
Your Site Network Partner for Executing Phase 1-4 Clinical Trials.
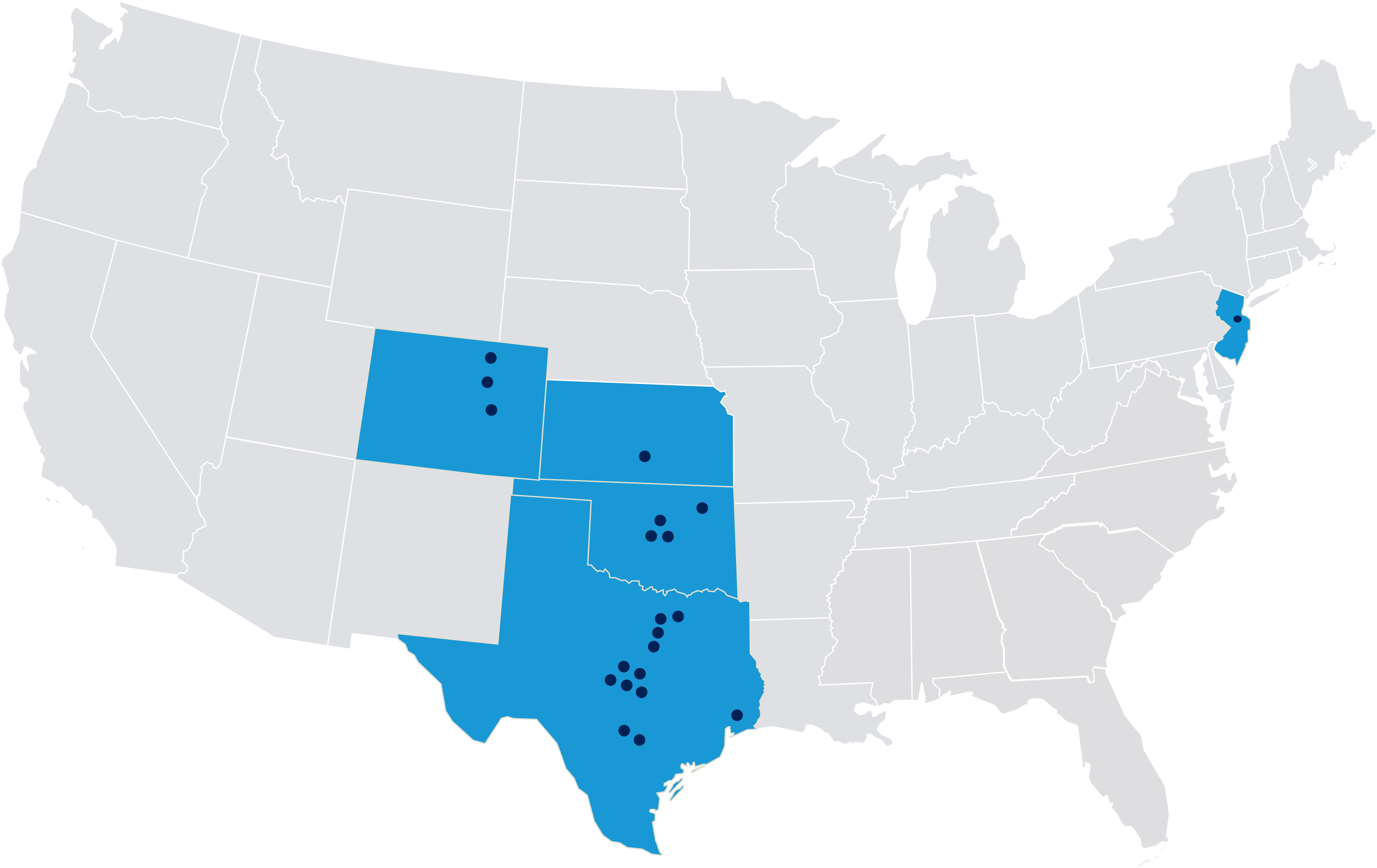
Welcome to Tekton Research, your premier partner for conducting Phase 1-4 clinical trials with unparalleled excellence. Our extensive investigator network covers key locations in Colorado, Kansas, New Jersey, Oklahoma, and Texas, showcasing exceptional expertise and unwavering commitment throughout the entire research process.
Backed by a team of accomplished principal investigators with diverse experience across therapeutic areas such as Endocrinology, Neurology, Dermatology, Rheumatology, and more, we successfully engage pediatric, adult, maternal, and elderly populations in our recruitment efforts.
Multi-Specialty
Therapeutic Areas

Hypertension
Hypercholesterolemia
Acne
Diabetes
Obesity
Osteoarthritis
Osteoporosis
Depression
Healthy Vaccine Studies
Treatment Vaccine Studies
Device Studies

Alzheimer’s disease
Amyloidosis
Depression
Epilepsy, idiopathic generalized epilepsy (IGE)
Essential Tremors
Ischemic stroke
Memory impairment
Migraine
Mild cognitive impairment (MCI)
Multiple sclerosis
Pain management
Parkinson’s disease
Restless legs syndrome
Psoriatic Arthritis
Lupus
Sjogren’s Syndrome
Ankylosing Spondyloarthritis
Osteoarthritis
Osteoporosis
Gout
Giant Cell Arteritis
Mixed Connective Tissue Disease
PMR
Scleroderma
Fibromyalgia

Type 2 Diabetes
Obesity
Hypothyroidism/Hyperthyroidism
Lipids
Hypertension
PCOS
Osteoporosis
Growth Hormone Deficiencies
Parathyroid Disorders
Thyroid Disorders
Pituitary Disorders
Adrenal Gland Disorders
Fatty Liver
Thyroid Disease
Obesity
Device Studies
Low Testosterone
Metabolic disorders
Osteoporosis
Influenza
Maternal
Newborns and Infants
Pediatrics, Adolescents
Geriatrics
Immunodeficient
MMR (Measles, Mumps, Rubella)
mRNA Vaccines
Norovirus
Parainfluenza
Pediatric Influenza
Pediatric Meningitis
Pertussis (Whooping Cough)
Plague
Pneumococcal Disease
Rabies
Respiratory Syncytial Virus
Smallpox
Staphylococcus
Tetanus
Type 3 Parainfluenza Virus (PIV3)/RSV Combo
Varicella
West Nile Virus
Yellow Fever
Zika Virus
Anthrax
Avian Influenza
Botulinum
C. difficile (C-diff)
Celiac Disease
Chikungunya
COVID-19 (SARS-CoV-2)
Cytomegalovirus
Dengue Fever
Diphtheria
Ebola
E. coli
Encephalitis
Epstein-Barr Virus (EBV)
Group B Streptococcus
Hepatitis B
Herpes Zoster (Shingles)
HIV
Human Metapneumovirus
Human Papillomavirus
Influenza (H1N1, H5N1, H7N1, H7N9)
Lyme Disease
Meningococcal Disease

Urine
Stool
Tissue
Saliva

Hypertension
Hypercholesterolemia
Acne
Diabetes
Obesity
Osteoarthritis
Osteoporosis
Healthy Vaccine Studies
Treatment Vaccine Studies
Device Studies

Actinic keratosis
Alopecia
Atopic dermatitis/eczema
Cellulite
Consumer products
Cosmetics
Fine Lines
Granuloma annulare
Hair loss
Hidradenitis suppurativa
Histology
Hyperhidrosis
Hyperpigmentation
Impetigo
Infections
Melanoma and skin cancers
Mid-face filler
Molluscum contagiosum
Nail fungus
Onychomycosis
Palmoplantar Pustulosis
Photoaging
Prurigo Nodularis
Pruritus and urticaria
Psoriasis
Purpura
Rosacea
Seborrheic dermatitis
Shingles
Surgical products
Tinea pedis/onychomycosis
Vitiligo
Warts
Wound care and scars

Cytomegalovirus
Endometriosis
Fetal health
Group B Streptococcus
Herpes
High-grade squamous intraepithelial lesion (HSIL)
Hot flash
Human papillomavirus (HPV) vaccines
Maternal vaccine
Minimally-invasive surgical procedures
Osteoporosis
Overactive bladder
Sexually-transmitted diseases
Urinary incontinence
Uterine fibroids
Vulvo-vaginal atrophy (VVA), dryness

Diagnostic Device Studies
Sample Collection Studies
Vaccine Studies

COVID-19 vaccine
Epilepsy
H5N1, H1N1 influenza vaccine
HPV vaccine
Human metapneumovirus (HMPV) vaccine
Infant formula
Influenza treatment
Lyme disease
Maternal vaccines
Measles, mumps, and rubella vaccine
Meningococcal vaccine
Migraines
Molluscum contagiosum
On- and off-season influenza vaccine
Parainfluenza virus vaccine
Pneumococcal vaccine
RSV vaccine
Tetanus, diphtheria, pertussis (Tdap) vaccine
Computed tomography (CT) scan
Echocardiography
Exercise stress testing
Magnetic resonance angiography (MRA)
Magnetic resonance imaging (MRI)
Multigated acquisition scan (MUGA) imaging
Positron emission tomography (PET) pharmacologic and stress/rest perfusion, as well as metabolic imaging
Pharmacologic stress testing
Stress echocardiography
Technetium 99m stress/rest imaging
Thallium 201 stress/rest imaging
Transesophageal echocardiography (TEE)
Cardiac MRIs
CPET (exercise stress testing)
Our Site Network
Locations
We Manage the Study Lifecycle from Start to Finish
Capabilities
- Startup Efficiencies
- Feasibility and Site Selection
- Patient Recruitment, Engagement, and Retention
- Quality Assurance
Startup Efficiencies
Tekton sites aim to be your first activated and enrolling sites. Our dedicated regulatory and contract teams collaborate with our clinical operations and recruitment departments, so we can efficiently activate our sites and begin screening upon green light.
Our clients have a single point of contact that oversees all start-up activities. Tekton Research utilizes metrics to ensure all action items remain on track to achieve target study timelines while keeping our clients informed about progress or barriers.
Feasibility and Site Selection
Tekton’s feasibility team and investigators are committed to providing reliable and timely feedback for site selection. Our sites can operate under a single confidentiality agreement or execute site-specific agreements based on client requirements. Our goal is to supply our clients with an enriched and accurate list of well-aligned, experienced sites that understand the indication and protocol complexities.
Tekton will propose PI’s and sites that are engaged, invested, and dedicated to meeting enrollment goals, providing a positive patient experience, and delivering quality data.
Patient Recruitment, Engagement and Retention
At Tekton, we begin considering the overall patient recruitment, engagement, and retention strategy once an initial feasibility questionnaire is received. Our patient recruitment department, operational team, and site staff work together, considering: patient populations, previous best practices, the complexities of the indication, inclusion and exclusion criteria, study design, and goals, to develop an efficient and effective patient recruitment strategy. A Tekton patient recruitment strategist collaborates with our clients and internal stakeholders to develop and execute these patient recruitment plans. We understand the importance of establishing a well-qualified patient pool as early as possible.
The Tekton team employs a variety of resources to pre-identify participants, including investigator databases, Tekton databases, community outreach and advocacy, local and centralized advertising campaigns, and AI technology. Our clinical site staff and dedicated recruitment specialists connect frequently with current study subjects as well as our Tekton proprietary databases, ensuring they remain interested, informed, and engaged. This communication improves the likelihood of future enrollment and dramatically improves patient retention for enrolling trials. Tekton collaborates with site staff, providers, and participants to actively involve patients in research, prioritizing their perspectives and needs, to provide the best patient experience possible.
Quality Assurance
Tekton sites adhere to SOPs and quality assurance initiatives in compliance with corporate and industry guidelines. Overseen by a dedicated QA team, Tekton conducts regular training sessions and updates within our quality management system.
Processes and practices are reviewed regularly to stay aligned with GCP, ICH, and FDA regulations. Being audit-ready at all times demonstrates Tekton’s commitment to excellence and regulatory compliance.
Our Team
Our staff operates cohesively as a team, fostering collaboration and unity. We emphasize a collaborative relationship with our clients, ensuring that we work together seamlessly to achieve shared goals.

Kip McKenzie

Corey Collins

Bruce Wakeman

Taryn Collett

Jeffrey Zucker

Timothy Ryan

Shannon Hall

Lisa Bjornestad

Blaire Graham

Stacy Whaley

Stacy Whaley
Read Our Latest News
Filter
Request a Meeting
"*" indicates required fields